ISSCR News

The ISSCR Advocates for Stem Cell Research on Capitol Hill
On a beautiful spring day in Washington, D.C., leaders from the ISSCR walked the halls of the U.S. Congress to inform legislators and their health policy staff about the progress and potential of stem cell research during the annual ISSCR Congressional Advocacy Day.

Remembering Connie Eaves
The ISSCR is deeply saddened to learn of the passing of Connie Eaves, a tremendous friend and globally renowned stem cell scientist. Dr. Eaves was a member of the Stem Cell Reports Editorial Board and served on the ISSCR Board of Directors from 2009-2015. Many current and former ISSCR leaders fondly remember Dr. Eaves and her impactful contributions to the Society and stem cell community.

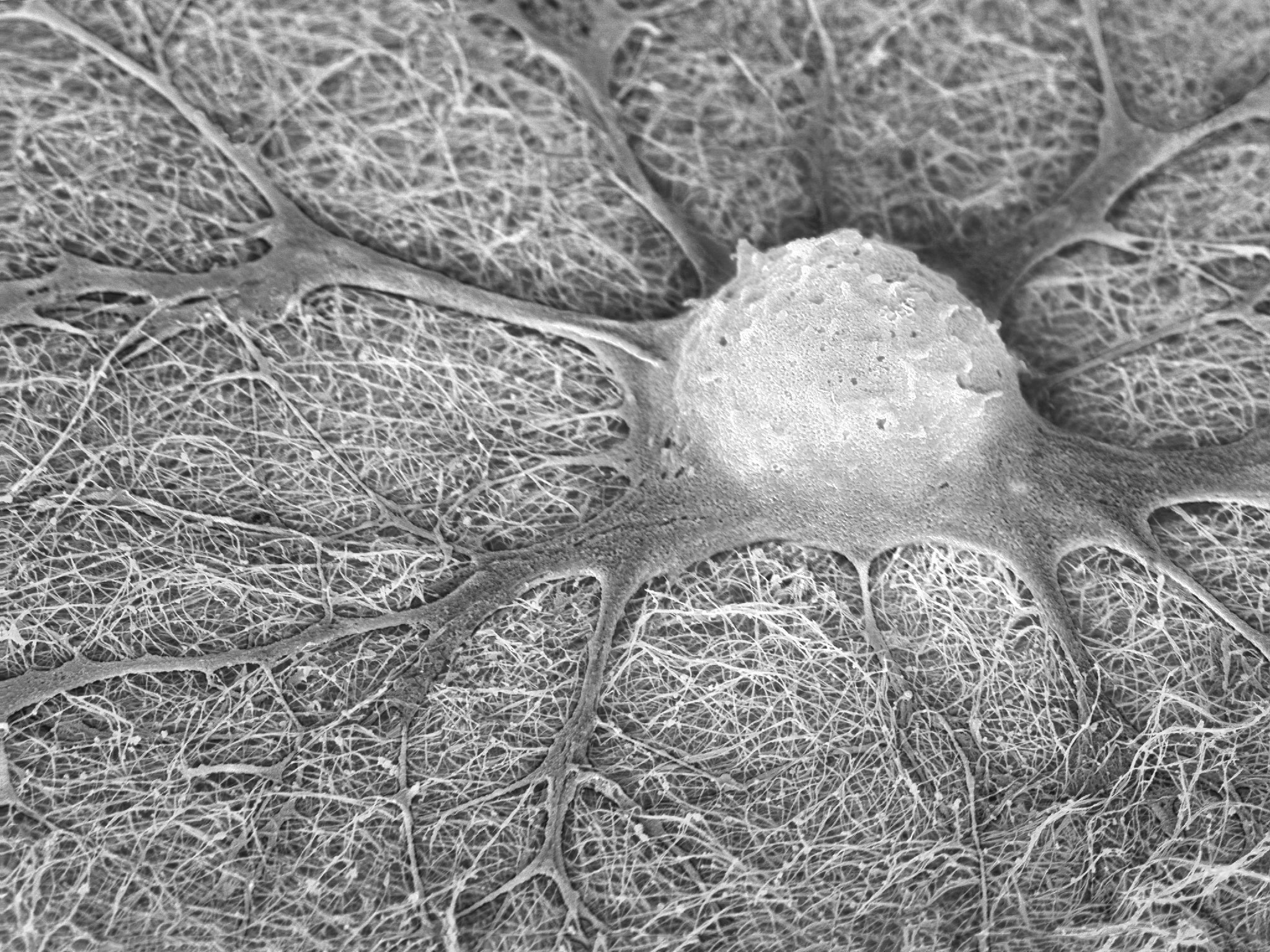
New Podcast Episode. Climbing the Scientific Mountain of Retinal Regeneration
Collectively, retinal degenerative disorders are a major cause of blindness worldwide. For example, one of the most common disorders is age related macular degeneration, which alone affects nearly 200 million globally. In humans, and other mammals, the loss of the retinal cells is an irreversible process. However, in some non-mammalian vertebrates like frogs and fish, retinal neurons can regenerate. This process is dependent upon Müller glia, which can re-enter the cell cycle and reprogram into neurogenic progenitors upon retinal injury or disease. Progress has been made in understanding the genetic program underlying these regenerative process, and proof-of-principle experiments in the adult mouse retina demonstrated that genetic programs in frog and fish can be coopted to induce neurogenesis in mammals. Our guests today have extended this research to genetically reprogram fetal or organoid-derived human Müller glia into retinal neurons. They will talk about this work, the background underlying it and its potential applications.

Member Spotlight: Elaine Fuchs, PhD
ISSCR is a wonderful international community of colleagues. Science has no borders and scientists are in many ways able to cut across political boundaries, and admire and learn from each other no matter where we live and work.

The ISSCR Provides Comments on FDA’s Draft Guidance for Potency Assurance for Cellular and Gene Therapy Products
On 5 March 2023, the ISSCR submitted comments on the Food and Drug Administration’s draft guidance for Potency Assurance for Cellular and Gene Therapy (CGT) Products. ISSCR appreciates FDA’s commitment to assuring the potency of human CGT products at all stages of the product lifecycle. This guidance from the FDA will help our members, who are at the forefront of research and innovation, in their work.
Receive ISSCR Press Releases
Sign up be a part of ISSCR’s media list. Media Contact: Kym Kilbourne, Director of Media and Strategic Communications
Subscribe to ISSCR News.
Each month, ISSCR delivers scientific, policy, and community to your inbox .
